New electronic signatures in Ziflow meet FDA record-keeping requirements. Streamline compliance today.
![]() Katie Oberthaler 29 Sep 2022 READ TIME: 2 MIN
Katie Oberthaler 29 Sep 2022 READ TIME: 2 MIN
New electronic signatures in Ziflow meet FDA record-keeping requirements. Streamline compliance today.
![]() Katie Oberthaler 29 Sep 2022 READ TIME: 2 MIN
Katie Oberthaler 29 Sep 2022 READ TIME: 2 MIN

Exceptional creative sometimes requires more than the right color palette or tagline. For creative teams in highly-regulated industries, meeting regulatory standards for maintaining creative assets is just as important as producing quality creative work itself.
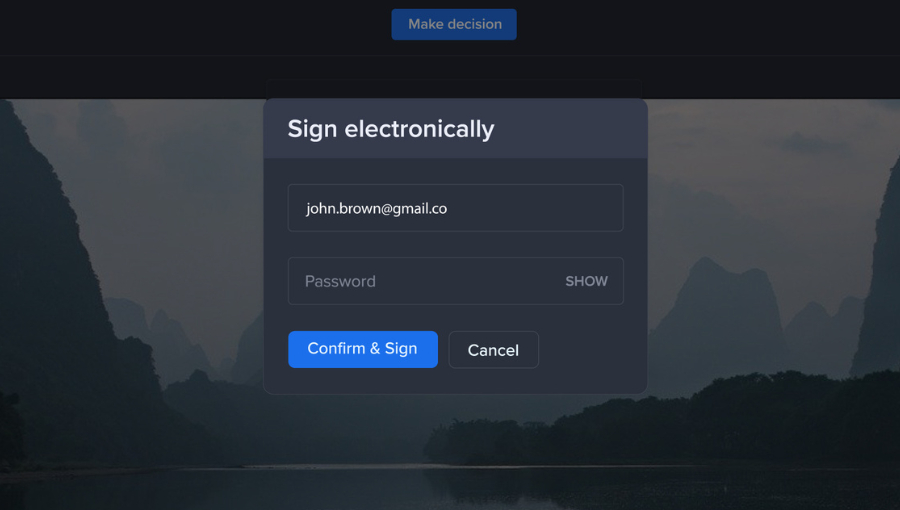
We’re excited to announce the launch of electronic signatures, a new feature in Ziflow that seamlessly enables creative teams to demonstrate compliance with FDA recordkeeping requirements.
Creative teams can now stipulate that review and approval decisions on assets must require an electronic signature in Ziflow. Electronic signatures form the cornerstone of compliance for industries regulated by FDA’s 21 CFR part 11 requirement, which obligates companies to prove that their digital records are trustworthy, reliable, and equivalent to paper records and physical handwritten signatures.
Assets approved in Ziflow using electronic signatures are now compliant with this requirement, taking care of this important step for the creative team.
Electronic signatures can be used to enforce this compliance for industries such as:
Creative teams working in these industries can now have the peace of mind that all marketing, branding and product materials follow the FDA’s guidelines for maintaining proper digital record keeping.
When a proof is created, such as a healthcare brochure, reviewers in Ziflow must provide credentials when making an approval decision with a “Confirm and Sign” prompt. This applies the reviewer’s signature to the asset.
Electronic signatures can be applied to assets and documentation such as:
Once an electronic signature has been applied to an asset, all decisions are marked as electronically signed on the proof’s activity log for complete visibility and auditing.
The activity report includes the signee’s information, the date and time of the action, and more. All records are made available in PDF format and can be viewed with a PDF Viewer. Ziflow maintains internal security and user processing controls to ensure that only authorized users with relevant permissions are able to inspect, review, and copy these electronic records.
Applying regulatory standards directly to creative assets and within the creative production workflows ensures that proper review and decisions are made on creative work by only the right people--and changes are traced accurately across all creative records. And eliminating regulatory headaches empowers creatives to focus on what they do best.
Increase accountability today with Ziflow’s electronic signatures capabilities. Read more about using electronic signatures feature here and get in touch with our team at hello@ziflow.com to learn more about how Ziflow’s ongoing innovation can help you deliver exceptional creative, faster.
See how Ziflow's collaborative proofing platform supports all of your campaigns and channels.

Keep every campaign compliant and on-brand with this marketing compliance checklist. Learn how to protect your brand and streamline approvals using Zi...

Compare the 10 best online proofing software tools for creative teams. Discover features, pros, cons, and how to choose the right platform for your wo...

Ready to choose a creative automation platform? Discover what to look for so your team can create faster, collaborate better, and produce consistent r...
Subscribe to our newsletter for product updates and industry insights.
GET STARTED
TEAMS
INTEGRATIONS
INDUSTRIES